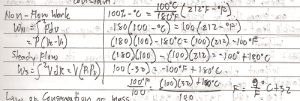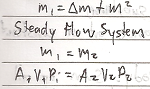Thermodynamics System and Surroundings
System is the term given to the collection of matter under consideration enclosed within a Boundary.
Surroundings is the region outside the boundary or the space and matter external to a system.
Closed System is a system in which there is a flow of matter through the boundary.
Isolated System is a system in which wither mass nor energy cross the boundaries and it is not influence by the surrounding.
Non-Flow Process is a process that take place in a closed system.
Steady Flow Process is a process that takes place in an open system in which the quantity of matter within the system is constant.
- Mass (M1) entering the system is equal to the sum of the stored mass ( _M) and the mass (M2) that leanes the system.
- Energy cannot be created nor be destroyed it can only be transferred from one form to another.
Second Law of Thermodynamics
- Heat cannot be transferred from cold body to an a heat body without an input of work. It similarly states that heat cannot be connected 100% into work. The Bottom line is that an engine must operate between a hot and cold reservoir.
Thrid Law
- The total entropy of pure substances approaches zero at the absolute thermodynamics temp. Approaches Zero. Also indicated that energy has different levels of potential B.
- Do work and that energy cannot naturally move from realm or lower potential to a realm of higher potential.
Zeroth Law
- When any two Bodies are in Thermal Equilibrium with the third body. They are in Thermal Equilibrium with each other.
Heat
- Form of energy associated with the kinetic random motion of large No. of molecules.
Sensible Heat
- Heat needed to change the temp. of the Body without changing its phase.
Latent Heat
- Heat needed by the body to change its phase without changing its temperature.
Latent Heat of Fusion of ICE = 144 13 tu/lb = 334 kj/ky
Latent Heat of Vaporization of Boiling H2O = 970 BN/bb = 2257 kj/ky
Entopy
- Is the measure Boyle’s Law of randomness of the transferred to a substance at a constant pressure.
Internal Energy
- Energy stored within the body sum of all kinetics energy of all its constituents particles plus the sum of all the potential energies of interaction among these articles if the temp is hold Constant the volume is inversely proportional to the absolute pressure.
Charles’s Law
- If the absolute pressure is held constant the volume is directly proportional to the absolute temp.
General Gas Law
- Even one of these laws states how one quantity varies with another if the third quantity remains unchanged but if the tree quantities chance simultaneously, it is necessary to combine these law’s in order
Temperature is an indication or degree of hotness and coldness and timeforce a measure of intensity of heat.
Six temp. Scales
- Celcius
- Farenheit
- Kelvin
- Rankine
- Reamur
- Ligem
Absolute Temperature
- Is the temperature measure from absolute zero